Despite a large amount of work that has concentrated on understanding colon tumor formation, we still do not know the full complement of molecular lesions that are individually necessary (and together sufficient) to cause colorectal cancer. Neither do we understand why some specific mutations that are relatively rare in other tumors are extremely common in colorectal cancer.
For the SYSCOL project, we propose to use the tools of systems biology to develop a quantitative and comprehensive model of colorectal tumor formation. The model will describe cellular pathways that contribute to tumor formation and explain in detail how the genetic disposition of an individual can activate expression of genes that drive uncontrolled cell growth and lead to cancer. This model will subsequently be used to find novel therapeutic targets, to guide genetic screening to identify individuals with elevated risk for developing colorectal cancer and to classify patients into sub-groups to select the treatment combination that is optimal for each patient (personalised medicine).
Specific aims of the SYSCOL project:
- Identify genetic markers for individual risk using genotyping and sequencing of germline DNA from sporadic and familial colorectal cancer cases and controls.
- Identify genes and regulatory elements that contribute to colorectal cancer cell growth.
- Use data from Aims one and two to develop a quantitative model for colorectal tumor formation.
- Apply the model for identification of high-risk individuals, for detailed classification of the disease and for identification of novel molecular treatment targets.
The proposed project is multidisciplinary and integrates basic research and clinical research based on patient material as well as computational and experimental research. The data generated and the methods and tools developed within the project will also lead to a better understanding of the complex networks of genes and gene regulatory systems behind colorectal cancer. We also expect the methods and tools to be applicable to the study of other multigenic disorders and thus the project will impact research not only on colorectal cancer but have broader implications on research, prevention and treatment of all multigenic disorders.
COLORECTAL CANCER IN BRIEF
The lower part of the digestive system is known as the colon, of which the last 15 cm is called the rectum. Cancer of the colon and rectum – colorectal cancer – begin as polyps (see adenoma) that grow on the inner lining of the large intestine.
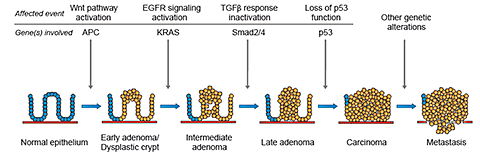
Most sporadic cases of colorectal cancer are believed to develop from benign adenomas (polyps) to carcinoma by the accumulation of genetic abnormalities (fig. 1A). However, only a small percentage of adenomas progress to carcinomas and the time period required for this development is lengthy, with a minimum of 5-10 years.
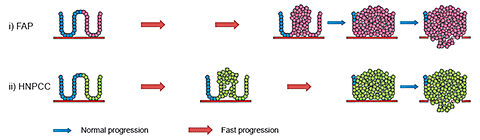
The majority of all colorectal cancers occur sporadically without any known cause, but certain groups of people have a predisposition to the development of cancer of the large intestine. These people may carry specific genetic mutations or have relatives with the condition. Approximately 15% of all colorectal cancer cases are familial, with the most common inherited conditions being familial adenomatous polyposis (FAP) and hereditary non-polyposis colorectal cancer (HPNCC) (fig. 1B). The familial cancers FAP and HNPCC both have an early onset, whereas the sporadic cases appear much later, around 60 years of age.
FAP is one of the best defined hereditary cancer syndromes. Individuals with FAP develop large numbers of polyps in the colon and rectum early in life. The median age at which colon
cancer is diagnosed in FAP patients is approximately 40 years, which is around 2 decades earlier than its occurrence in the general population. Patients with FAP have a lifetime risk of the development of colon cancer that approaches 100%.
HNPCC is a familial disorder characterized by a high incidence of colon cancer without the excessive polyps identified in FAP. Patients with HNPCC develop colon cancer at an early age and it could account for up to 6% of colon cancers. HNPCC arises because of mutations in the mismatch-repair genes (hMSH2, hMLH1, hPMS1 and hPMS2), and this leads to instability in the DNA.
SCREENING AND TREATMENT
Colorectal cancer can take many years to develop and early detection of colorectal cancer greatly improves the chances of a cure. Screening has the possibility to detect cancer, polyps and smaller lesions in the colon. If the screening reveals a problem, diagnosis and treatment can occur promptly. There are several screening methods available, including a rectal exam, fecal blood test and colonoscopy.
The treatment of choice depends on how far the cancer has progressed. Surgery is the primary treatment for removal of polyps and/or cancer tumours. Chemotherapy can be used to limit spreading of the cancer to other parts of the body. Radiation therapy is less common in colon cancer, but is sometimes used to treat rectal cancer, as the rectum does not move as much as the colon.
Read more about screening methods and treatments here.
http://www.cancer.org/Cancer/ColonandRectumCancer/OverviewGuide/colorectal-cancer-overview-detection
http://www.cancer.gov/cancertopics/pdq/treatment/colon/Patient/page1
THE GENES AND EVENTS INVOLVED IN COLON CANCER DEVELOPMENT
There are two key types of genes involved in cancer development:
- Tumour suppressors – whose protein products have a repressive effect on cell proliferation or promote apoptosis (programmed cell death), or both.
- Oncogenes – whose protein products, when activated, cause uncontrolled cell proliferation or prevent cells from undergoing apoptosis.
Non-functional tumour suppressors or activated oncogenes can cause the development of cancer.
THE APC AND THE WNT SIGNALLING PATHWAY
The adenomatous polyposis coli (APC) gene is a tumour suppressor gene, i.e. a gene preventing uncontrolled cell growth that could lead to the formation of a cancer tumour. The APC is a protein involved in the Wnt signalling pathway, which is a network of proteins with prominent roles in embryogenesis, cell differentiation and cancer.
The Wnt proteins bind to receptors on the cell surface. This initiates a cascade of events that ultimately lead to the stabilization of the β-catenin protein, an intracellular signalling molecule. β-catenin can then enter the cell nucleus and assist in activating transcription of Wnt target genes that are involved in cell proliferation.
The APC protein is part of a complex that promotes degradation of β-catenin, thereby inhibiting the transcription of several genes involved in cell proliferation. Mutations in the APC gene leading to loss of APC protein function is one of the earliest events in colorectal tumorigenesis. Approximately 85% of all colorectal tumours exhibit loss of APC function.
KRAS AND EGFR SIGNALLING
The epidermal growth factor receptor (EGFR) pathway is a complex signaling cascade that is involved in the development and progression of cancer. Like in the Wnt signaling pathway, ligands bind to the EGFR on the cell surface and initiate a cascade of events in the cell that in this case lead to regulation of genes that control cell cycle progression.
KRAS (Kirsten rat sarcoma), an oncogene and a member of the RAS family of signal-transductors, regulates downstream proteins at an early stage of this cascade. When KRAS is mutated it stimulates cell proliferation, an essential step in cancer development. In the development of colorectal cancer however, timing of the mutation is important. Only if it occurs after a previous APC mutation can it lead to the formation of a tumour.
SMAD2/4 AND THE TGFβ RESPONSE
Transforming growth factor beta, TGFβ, acts as an anti-proliferative factor in epithelial cells. It can halt the cell cycle to stop proliferation, induce differentiation and induce apoptosis via the SMAD signalling pathway. SMAD proteins transduce signals from TGFβ ligands at the cell surface to the nucleus where they activate the transcription of genes that trigger apoptosis. When components of the TGFβ signalling pathway, e.g. Smad 2 and/or 4 are mutated, cell proliferation is uncontrolled and can lead to the development of cancerous tumours.
P53
The p53 protein is a key player in sensing DNA damage, DNA repair and apoptosis. Mutations in p53 are seen in 50-70% of carcinomas, but not in adenomas, suggesting that they occur quite late in the development of colorectal cancer. The mutations in p53 cause failure of apoptotic mechanisms and deregulate cell proliferation, thereby leading to genomic instability and malignant progression. Click on the pictures for larger versions.
Progression from normal epithelium to colon cancer
A. Sporadic colorectal cancer
B. Familial colorectal cancer
FIGURE LEGEND
Progression from normal epithelium to colorectal cancer requires an accumulation of mutations in particular genes that affect the balance between proliferation and apoptosis.
A. The steps in development of sporadically occurring cancer in a normal colon epithelium. However, not all colorectal tumours exhibit all the mutations shown in the above figure.
B. Individuals with an existing cancer predisposition.
i) FAP: germline inactivation of one APC allele. Adenoma formation is faster, but progression from adenoma to carcinoma has the same rate as sporadic colorectal cancer.
ii) HNPCC: germ line inactivation of one allele of either of the mismatch repair genes MSH2 or MLH1 in combination with somatic inactivation of the other allele leads to an increase in the mutation rate, which in turn speeds up the adenoma to carcinoma progression.
Adapted from Davies, R. J., et al. 2005. Colorectal cancer screening: prospects for molecular stool analysis, Nature Review Cancer 5:199-209
REFERENCES
- Fearon, E. R and Vogelstein, B. A genetic model of tumorigenesis. Cell 61:759-767, 1990
- Bienz, M and Clevers, H. Linking colorectal cancer to Wnt signaling. Cell 103:311-320, 2000
- Taipale, J, and Beachy , P.A. The Hedgehog and Wnt signalling pathways in cancer. Nature 411:349-354, 2001
- Slaby, O et. al. Micro-RNAs in colorectal cancer: translation of molecular biology into clinical application. Molecular cancer 8:102, 2009
- Vries, R. G. J, Huch, M and Clevers, H. Stem cells and cancer of the stomach and intestine. Molecular oncology 4: 373-384, 2010
- Fearon, E. R. Molegular genetics of colorectal cancer. The Annual Review of Pathology: Mechanisms of disease 6:479-507, 2011
COLORECTAL CANCER ON THE WEB
http://www.cancer.gov/cancertopics/pdq/treatment/colon/Patient/page1